The formula of a synthesis reaction is as follows. Single displacement reactions take the form.

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
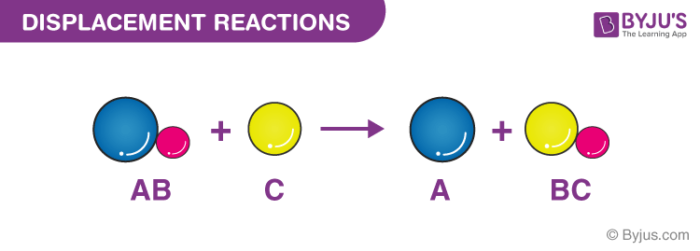
A single-displacement reaction also known as single replacement reaction or exchange reaction is a chemical reaction in which one element is replaced by another in a compound.
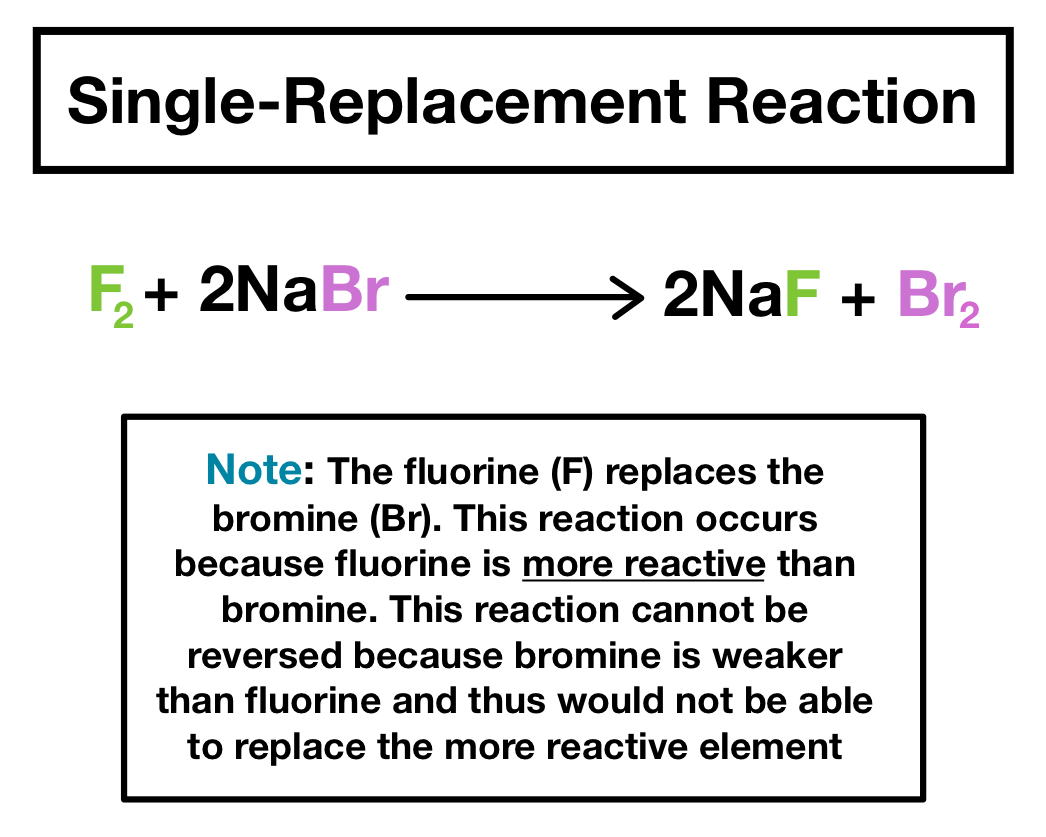
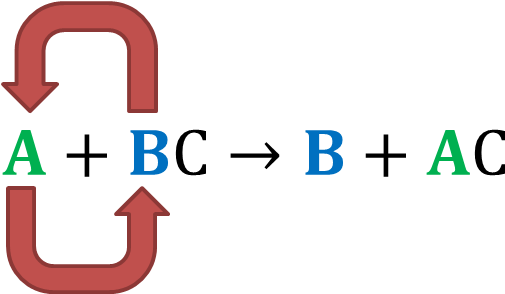
. It can be represented generically as. A BC AC B displaystyle ce A BC - AC B where either. During the reaction A replaces B forming the product compound AC and releasing the less reactive element B.
A BC B AC. A displaystyle ce A and. ABC B AC.
This can either be in the form of a single replacement reaction or a double replacement reaction. A single-replacement reaction sometimes referred to as a single-displacement reaction is a chemical reaction in which one element is substituted for another element in a compound generating a new element and a new compound as products. ZnCuCl2 Cu ZnCl2.
X Y XYAs it is possible to warn X and AND are two different chemicals that combine to form a new product XY. In this reaction one cation replaces another one from its solution. There are two types of single replacement reactions.
A cation is a positively charged ion or metal. Cation replacement reaction A Cation Replacement Reaction. A chemical reaction in which an element replaces one element in a compound.
If you ask a child to draw a house he or she likely will not draw a modern slider window a casement window or a picture windowAlmost certainly they will draw a box with a gable roof and a single-hung or double-hung window. A single-replacement reaction replaces one element for another in a compound. A single-displacementreaction also known as a single-replacementreaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that.
Looking for single-replacement reaction. In a double replacement reaction there is interchanging of ions or atoms between the reactants. A double-replacement reaction exchanges the cations or the anions of two ionic compounds.
In single replacement reactions one ion or an atom of an element is displaced by another ion or atom discussed in detail below. Single-hung and double-hung windows are two of the most basic classic and timeless styles of windows you can install in your house. All metal displacement reactions are cation replacement reactions.
Displacement reactions can be further classified into single replacement reaction and double replacement reaction. Any view expressed on the newspapers editorial pages is. Espoused hitched married wedded.
A metal replaces another metal that is in solution. Neutralization precipitation and gas formation are types of double. When chlorine is added in its gaseous form or as a gas dissolved in water to the solution of sodium.
The periodic table or an activity series can help predict whether single-replacement reactions occur. Double replacement reactions are also called double replacement reactions double displacement reactions or metathesis reactions. A double replacement reaction is a type of chemical reaction that occurs when two reactants exchange cations or anions to yield two new products.
Also wed 2 belonging only to the one person unit or group named. A single replacement reaction aka single displacement reaction will occur if M 1 cation is less reactive than M 2The reactivity order corresponds to the reactivity series of the metals. It is also known as a single-replacement reaction.
A single-displacement reaction also known as a single-replacement reaction is a type of chemical reaction where an element reacts with a compound and takes the place of another element in that. Chemical reaction involving ions where one element is replaced by another in a compound. Predicting and determining the products using the reactivity series.
A single-displacement reaction is a chemical reaction where one reactant is exchanged for one ion of a second reactant. A replacement reaction is a type of chemical reaction in which one element replaces another in a compound. Up to 24 cash back Single-replacement reaction is also called a single-displacement reaction this type of reaction is a oxidation-reduction chemical reaction.
The historical figure who is considered to be the first to give real and conscious form to the synthesis reactions is the German chemist Friedrich Wholer 1800 1882 who achieved this result from. This type of reaction is when an ion or an element moves out of one compound and into a different compound. Find out information about single-replacement reaction.
A single replacement reaction occurs when an element reacts with a compound to produce a new element and a new compound. Classification of single displacement reaction. An example of a single replacement reaction occurs when potassium K reacts with water H 2 O.
Definition of single replacement or single displacement reactions. In this equation A represents a more reactive element and BC represents the original compound. A single displacement reaction which is also called as single replacement reaction is a kind of oxidation-reduction chemical reaction when an ion or element moves out of a compound ie one element is replaced by the other in a compound.
This indicates how strong in your memory this concept is. In a single replacement reaction one of the reactants is more reactive than the other which results in the formation of a product that is more stable.

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com
Download Single Replacement Reaction Single Replacement Definition Png Image With No Background Pngkey Com

Single Replacement Single Displacement Reaction
Displacement Reactions Definition Types Single Double Examples

Single Replacement Reaction Definition And Examples

Single Displacement Reaction Definition Examples Video Lesson Transcript Study Com

Chemical Reactions 2 Of 11 Single Replacement Reactions An Explanation Youtube
0 comments
Post a Comment